This blog post will introduce general process control methodlogy with additional tools such as control plan, value stream mapping, Certificate of Analysis and SIPOC diagram.
Introduction
Process control deployment is a common methodology to ensure corporate’s manufacturing process is in a stable and consistent state. The formulation is to ensure all potential risks can be controlled and mitigated.
The following diagram will demonstrates the general steps how process control is formulated from process flow into actual records.
The detailed stages are listed below:
- Before understanding how to control the process, generate a process flow diagram to have an overview and identify respective stations. This helps people who do not know the process to have a quicker understanding of production environment before going further.
- Use process/design FMEA to identify potential risk during the design phase or process failures. In design FMEA, core element of failure shall be based on the potential risk generated from the product’s function failure. While process FMEA focuses more on the failure mode during the production from incoming raw material up to finished products.
- After consider and evaluated all potential risks, Control Plan will be list respective process/product parameters which needs to be controlled and recorded. Control Plan shall also have the recording method of parameters and respective countermeasures when abnormality occurs.
- Since production process needs documented procedure, record forms indicates the process evidence is documented and ensure the process is well recorded for reference.
SIPOC Diagram
In addition to general process control, SIPOC diagram is also applied to assist process control designers to consider more details. And the following definition will explain each letter of SIPOC stand for before letting the users to consider how to establish process control at each stage.
The following example will demonstrate how the SIPOC relation works for chemical mixing industries.
Value Stream Mapping (VSM)
Value Stream Mapping (VSM) is generally used to illustrate product realization processes starting from raw material to the phase where the final goods are delivered to customers. Value stream map also ensures the detailed steps are breaking down to a sequence of flow of information and resource within the process.
When evaluating value stream mapping, the following points shall be considered:
- Costumer focused (end to end process)
- Helps to visualize process flow (starts, stops and idle time)
- Identify potential waste, resource allocation and operation time improvement.
- Have the current and future process maps step by step.
- Information separated with process physical flow (yield, operators qty, cycle time, downtime...etc.)
The VSM example (see below) will illustrate the tire manufacturing flow from raw material to customer end.
Control Plan
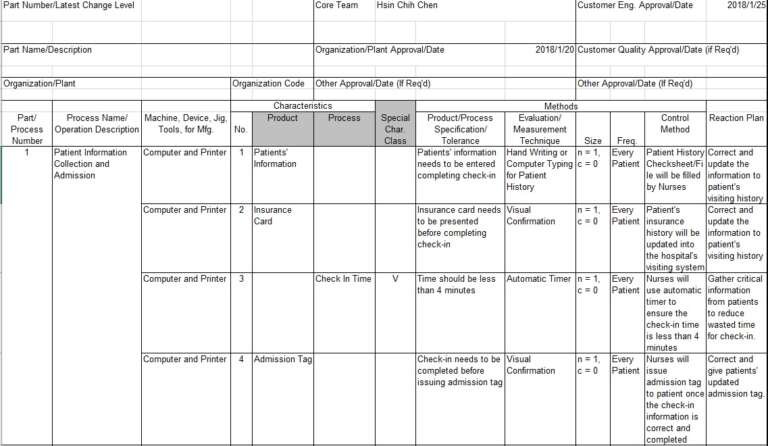
Control plan is generally used to have a detailed process flow layout with control methods respectively, it can detail the details of each process step/parameter’s control method, inspection frequency along with contingency plans in case the control fails.
The following steps are the general rule of thumb for control plan setup.
When establishing control plan, the following items (with respective product/process nature) should be considered before finalizing control methods
- Process Name / Operation Description (with correct process number ordered)
- Involved Equipment in Process
- Process / Product Characteristics
- Process / Product Specification (USL/LSL)
- Measurement Technique
- Sample Size & Frequency
- Control Method and Contingency Plan
The following example summarizes the general control plan layout.
Certificate of Analysis (CoA)
Certificate of Analysis (CoA) is an evidence of supplier’s final product quality check and can be applied to incoming quality control (IQC)’s reference, and it’s a critical contract between supplier and manufacturing companies’ quality agreement.
The following steps are the general rule of thumb for control plan setup.
Throughout the Certificate of Analysis formulation, the following elements shall be considered in order to fully assure the Certificate of Analysis is on the full coverages between the company and suppliers.
- Evaluate the raw material’s special characteristics and respective acceptance criteria before evaluating the specification. The appropriate testing method shall also be decided, whether it’s from an international standard such as ASTM or JIS.
- Trial data between supplier’s trial and RD’s trial shall be cross-verified before finalizing the acceptance criteria and testing method’s reference.
- This will be the official contract agreement between company and suppliers. Generally, items such as testing characteristics (whether it’s mechanical or chemical properties), reference testing standard and acceptance criteria shall be recorded within the supplier quality agreement. RD, SQE and supplier representatives shall sign it to make the supplier quality agreement official.
The following graph will summarize the roles and responsibilites to formulate the CoA.